
Corrosion is the natural, gradual degradation of metals as they chemically react with their surrounding environment. It is an electrochemical process that transforms refined metals into more chemically stable forms such as oxides, hydroxides, or sulfides. This transformation leads to the deterioration of the material’s properties, including strength, appearance, and permeability, and can eventually cause structural failure.
At its core, corrosion involves a redox (reduction-oxidation) reaction:
Oxidation: The metal loses electrons and forms positive metal ions.
Reduction: A substance in the environment (typically oxygen in water or air) gains the electrons.
For most types of corrosion to occur, the following elements must be present:
A metal (e.g., iron, aluminum, steel)
An electrolyte (e.g., water containing salts or acids)
An oxidizing agent (usually oxygen)
An electrical path (for electron flow between anodic and cathodic sites)
Check out the visual illustration below showing the basic corrosion process:
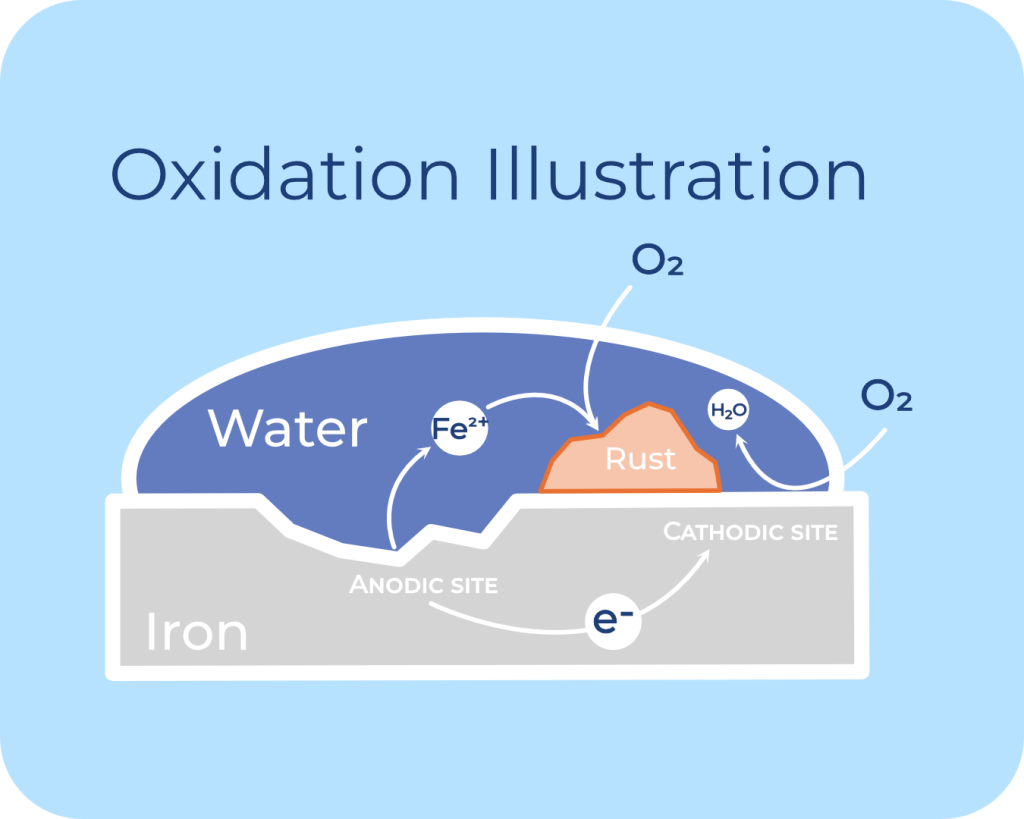
When selecting or designing fasteners for industrial or structural applications, understanding the major types of corrosion they may face is critical. Fasteners are often exposed to a range of environmental conditions, and their failure can compromise the integrity of an entire structure. Here’s an overview of the major types of corrosion relevant to fasteners: general corrosion, pitting corrosion, crevice corrosion, stress corrosion cracking, intergranular corrosion, fretting corrosion, galvanic corrosion, and hydrogen embrittlement.
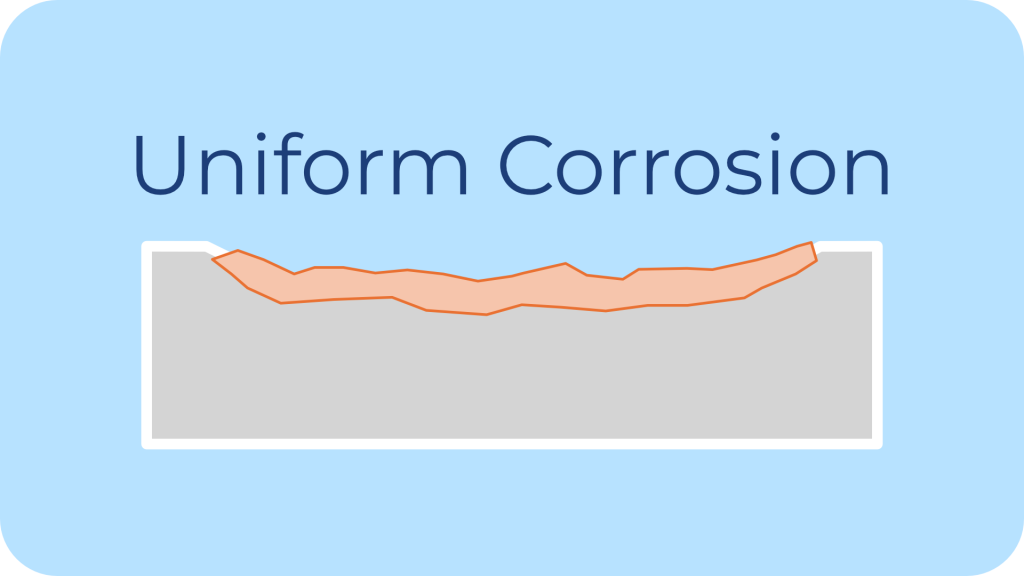
Description: Uniform corrosion is also known as general corrosion, it occurs evenly across the surface of a metal when it reacts with its environment (typically oxygen and moisture).
Common Environments: It occurs in humid, marine, or industrial atmospheres.
Impact on Fasteners: It leads to gradual thinning and weakening of the fastener material, potentially leading to structural failure over time.
Description: Pitting Corrosion is highly localized form of corrosion that results in small, deep pits or holes in the metal surface.
Common Environments: It occurs commonly in Chloride-rich environments, such as coastal areas or where de-icing salts are used.
Impact on Fasteners: It can lead to failure from a small but deep penetration that weakens the structure.
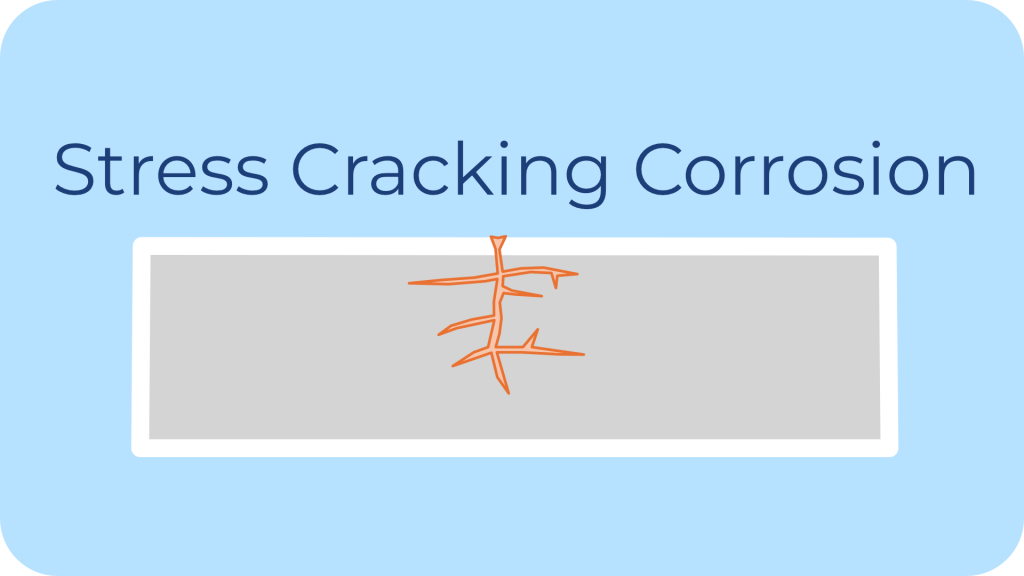
Description: Stress corrosion cracking is induced from the combined influence of tensile stress and a corrosive environment.
Common Environments: Typically this corrosion occurs in specific alloys (e.g., stainless steels, high-strength alloys) in chloride environments or caustic environments.
Impact on Fasteners: It can lead to sudden and catastrophic failure, especially in high-stress applications like pressure vessels or structural joints.
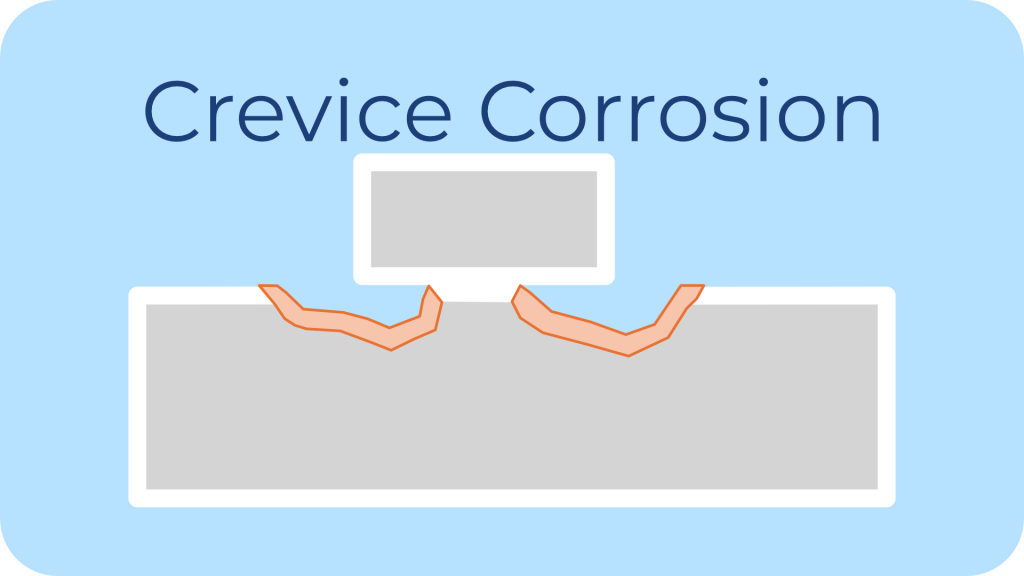
Description: Crevice corrosion is localized corrosion occurring in shielded areas such as under washers, gaskets, or between mating surfaces.
Common Environments: These are stagnant microenvironments with limited oxygen but access to chlorides or other corrosive agents.
Impact on Fasteners: Crevice corrosion initiates beneath the head of a bolt or between the bolt and mating surface, often undetectable until significant damage has occurred.
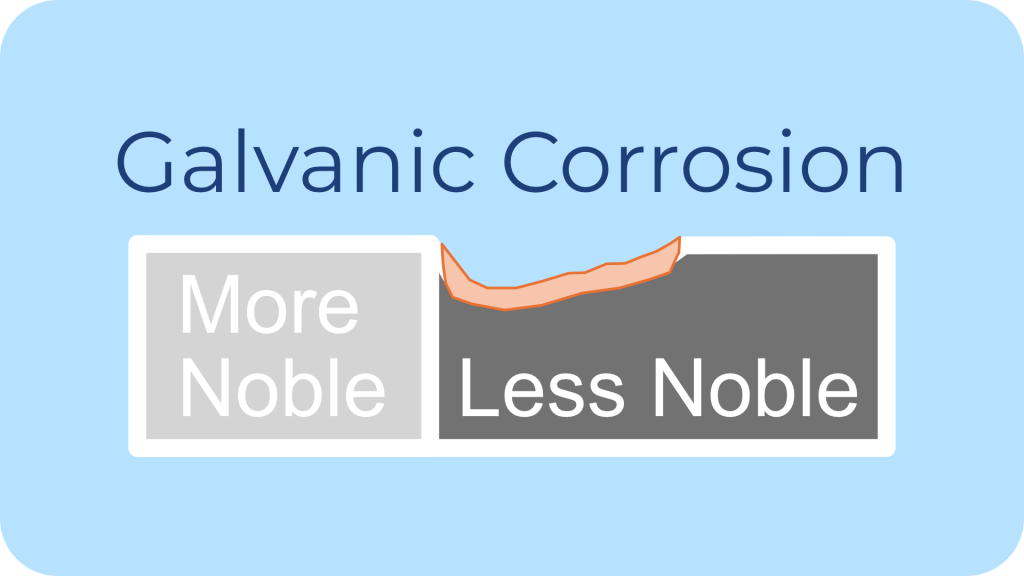
Description: Galvanic corrosion occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte (like water), leading to accelerated corrosion of the more anodic (less noble) metal.
Common Environments: The most common environments are marine and outdoor settings with varying metal types.
Impact on Fasteners: Fasteners often act as either the cathode or anode, and if the material pairing is not well-selected, one component corrodes much faster.
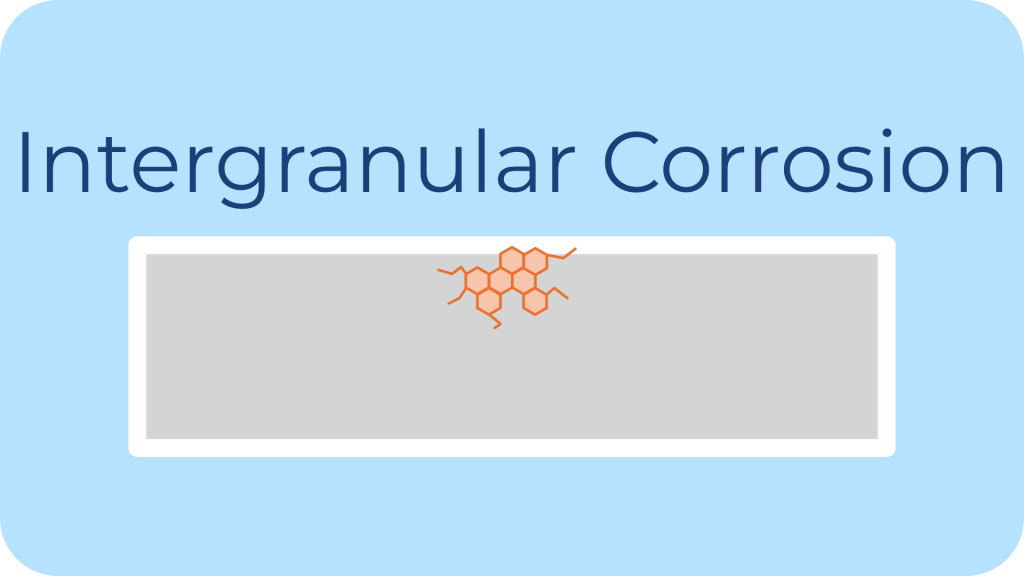
Description: Intergranular corrosion attacks the grain boundaries of the metal, often due to poor heat treatment or improper welding.
Common Environments: Environments where chromium-depleted grain boundaries occur in stainless steels typically cause intergranular corrosion.
Impact on Fasteners: Intergranular corrosion weakens the material structure internally, potentially leading to failure under stress.
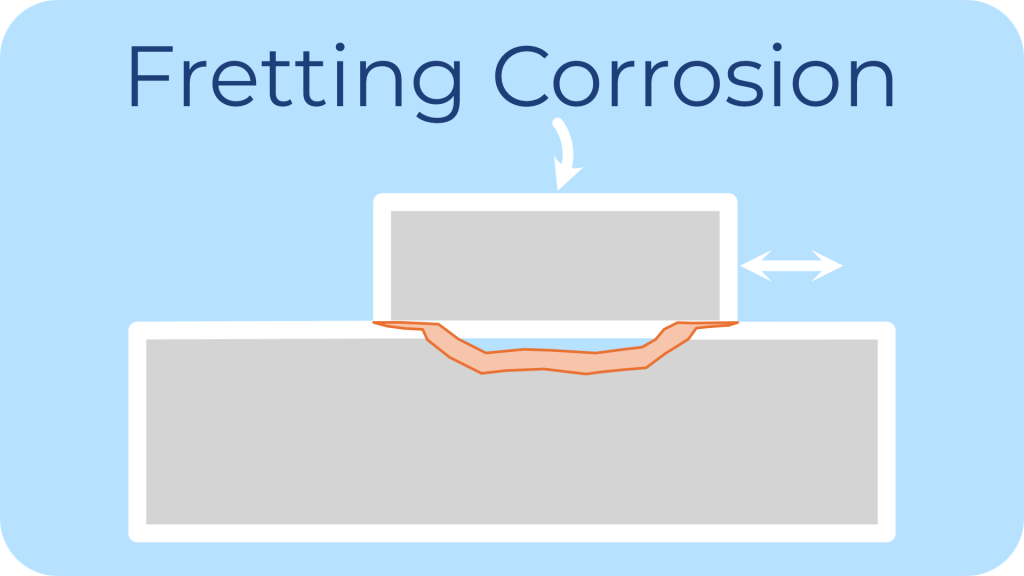
Description: Fretting corrosion is caused by repeated small movements between two contact surfaces under load, resulting in wear and oxidation.
Common Environments: Vibrating assemblies, mechanical joints are the most common environments which cause fretting corrosion.
Impact on Fasteners: Fretting corrosion leads to loss of material and can increase susceptibility to other corrosion types.
Corrosion has significant economic, safety, and environmental impacts. Structural failure in buildings, bridges, pipelines, and aircraft can lead to serious injury or death. Contamination of products (e.g., in food and pharmaceutical industries) can lead to serious illness. Cost of prevention, repair, and replacement leads to billions annually. Safety hazards including explosions, leaks, or collapses can lead to failures in our infrastructure and serious injury or death.
Reach out to us to ask us about our high nickel alloy corrosion, galling or heat resistant fasteners. Begin your quote today!